
It is difficult to overestimate the importance of observational skills in Science. Keen observations underpinned much of the work of the founders of Science and Art from Aristotle and beyond. Perhaps the greatest advocate of observation was the polymath, and all round genius, Leonardo da Vinci. Leonardo’s notebooks incorporate his detailed musings and advice on the primacy of visual observation. For example, before starting a portrait, he believed that an artist could only capture the true image of the subject with a full appreciation of form, colour, light (and critically its reflection in the composition): not forgetting a total mastery of draughtsmanship! Observations of biological specimens, such as the fine illustrations produced by botanists and the microscopic images in Robert Hooke’s Micrographia, have informed key concepts in Biology.
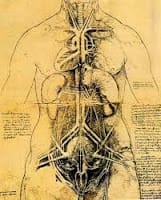
Leonardo was also fascinated by the formation of images in mirrors and the connection between the anatomy of the vertebrate eye, which, as he put it poetically: provides our “window on the soul”. In fact, the image of a face in a mirror, captures the subject in full colour, in three dimensions, with perspective. Leonardo’s interest extended to investigations into the inversion of images in experiments with dissected, vertebrate eyes. And, at the whole organism level, Leonardo’s landmark drawings of dextrocardia (below), a birth abnormality in which the heart is positioned on the right, rather than the left side of the chest, are splendid examples of his keen observational skills. Interestingly, the genetic origin underlying this “mirror-image” anatomy, which has more recently been observed in extrimis by drosophila geneticists as situs inversus totalis, are mutant alleles of a myosin gene.
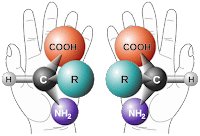
These early observations on the “handedness” and symmetry of living organisms can also be found at the molecular level, which is an observation that is often attributed to Louis Pasteur. Before we go any further, let’s begin with a definition of chirality. The term is derived from the Greek word χειρ (kheir) for “hand”. An object is said to be chiral if that object and its mirror image are non-superimposable, just like your right and left hand. In fact, just as our hands are chiral, so too are gloves: with your left hand palm facing down and horizontal, overlay your right hand: they cannot be superimposed. The natural chirality of our hands has in turn, determined the design rules for gloves. In a similar way, certain molecules are chiral, and as a conseuence, those molecules that interact with them are also chiral.

It was Louis Pasteur, known to most as the father of microbiology, paved the way for our understanding of the central role of chirality in Biology. Early in his career, working with tartaric acid crystals, he observed that mirror-image crystalline forms could be isolated, each with the distinctive capability of rotating plane polarized light in different directions: left or right. (This optical phennomenon had been described empirically by Jean Baptiste Biot, in studies of solutions of tartaric acid salts). This connection between the incident light and the molecular conformation of a relatively pure compound, paved the way for an understanding of chirality.
One of the most studied examples of molecular chirality are proteins, which are composed of one or more chains of amino acids (drawn from a menu of 20 types). All of the 20 naturally occurring amino acids (for accuracy Proline is an imino acid, and there are, therefore 19 true amino acids), have the same chirality, designated L, for laevorotatory. So, a solution of, for example L-Histidine, will rotate plane polarised light to the left (or counter-clockwise). Similarly, the amino acid L-Lysine if reflected in a mirror would appear as ɘniƨγ⅃. The tetra-valent nature of carbon, is a fundamental feature of the chemistry of all living organisms, and so carbon chemistry goes hand in hand with chirality: sorry!
- Leonardo Da Vinci (Florence, 1452-1519)
- Louis Pasteur (Paris, 1822-1895)
- Jean Baptiste Biot (Paris, 1774-1862)
- Sir Rovert Hook (Oxford; London, UK, Cambridge, UK, 1635-1703)
- Charles Darwin (Shrewsbury/Kent 1809-1882)
This and subsequent posts will be illustrated with images created by James Hornby, from whom you may request permission to use the images freely for educational purposes.
The author would like to thank Entropix for supporting the development of this Blog site.
